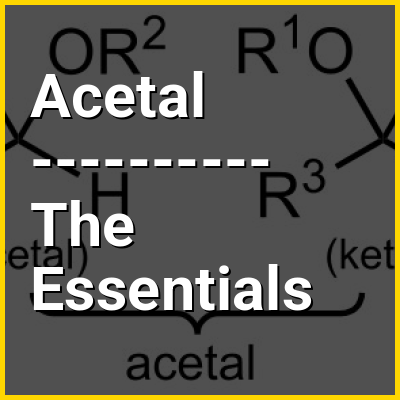
In organic chemistry, an acetal is a functional group with the connectivity R2C(OR')2. Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.
The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place of an R on the central carbon). The IUPAC originally deprecated the usage of the word ketal altogether, but has since reversed its decision. However, in contrast to historical usage, ketals are now a subset of acetals, a term that now encompasses both aldehyde- and ketone-derived structures.