Wendell Meredith Stanley (August 16, 1904 – June 15, 1971) was an American biochemist, virologist and Nobel laureate. Stanley's work contributed to lepracidal compounds, diphenyl stereochemistry, and the chemistry of the sterols. His research on the virus causing the mosaic disease in tobacco plants led to the isolation of a nucleoprotein which displayed tobacco mosaic virus activity.

>>>PUT SHARE BUTTONS HERE<<<
- 👉 Stereochemistry in the context of Wendell Meredith Stanley
- Stereochemistry in the context of Reaction mechanism
- Stereochemistry in the context of Equilateral triangle
- Stereochemistry in the context of Pseudoelement symbol
- Stereochemistry in the context of Stereoisomerism
- Stereochemistry in the context of Jacobus Henricus van 't Hoff
- Stereochemistry in the context of Epimer
- Stereochemistry in the context of Mathematical chemistry
- Stereochemistry in the context of Cyclic compound
Stereochemistry in the context of Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each.
Stereochemistry in the context of Equilateral triangle
An equilateral triangle is a triangle in which all three sides have the same length, and all three angles are equal. Because of these properties, the equilateral triangle is a regular polygon, occasionally known as the regular triangle. It is the special case of an isosceles triangle by modern definition, creating more special properties.
The equilateral triangle can be found in various tilings, and in polyhedrons such as the deltahedron and antiprism. It appears in real life in popular culture, architecture, and the study of stereochemistry resembling the molecular known as the trigonal planar molecular geometry.
Stereochemistry in the context of Pseudoelement symbol
The skeletal formula, line-angle formula, bond-line formula or shorthand formula of an organic compound is a type of minimalist structural formula representing a molecule's atoms, bonds and some details of its geometry. The lines in a skeletal formula represent bonds between carbon atoms, unless labelled with another element. Labels are optional for carbon atoms, and the hydrogen atoms attached to them.
An early form of this representation was first developed by organic chemist August Kekulé, while the modern form is closely related to and influenced by the Lewis structure of molecules and their valence electrons. Hence they are sometimes termed Kekulé structures or Lewis–Kekulé structures. Skeletal formulas have become ubiquitous in organic chemistry, partly because they are relatively quick and simple to draw, and also because the curved arrow notation used for discussions of reaction mechanisms and electron delocalization can be readily superimposed.
Stereochemistry in the context of Stereoisomerism
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.
Stereochemistry in the context of Jacobus Henricus van 't Hoff
Jacobus Henricus van 't Hoff Jr. (Dutch: [vɑn (ə)t ˈɦɔf]; 30 August 1852 – 1 March 1911) was a Dutch physical chemist. A highly influential theoretical chemist of his time, Van 't Hoff was the first winner of the Nobel Prize in Chemistry. His pioneering work helped found the modern theory of chemical affinity, chemical equilibrium, chemical kinetics, and chemical thermodynamics. In his 1874 pamphlet, Van 't Hoff formulated the theory of the tetrahedral carbon atom and laid the foundations of stereochemistry. In 1875, he predicted the correct structures of allenes and cumulenes as well as their axial chirality. He is also widely considered one of the founders of physical chemistry as the discipline is known today.
Stereochemistry in the context of Epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization is the interconversion of one epimer to the other epimer.
Stereochemistry in the context of Mathematical chemistry
Mathematical chemistry is the area of research engaged in novel applications of mathematics to chemistry; it concerns itself principally with the mathematical modeling of chemical phenomena. Mathematical chemistry has also sometimes been called computer chemistry, but should not be confused with computational chemistry.
Major areas of research in mathematical chemistry include chemical graph theory, which deals with topology such as the mathematical study of isomerism and the development of topological descriptors or indices which find application in quantitative structure-property relationships; and chemical aspects of group theory, which finds applications in stereochemistry and quantum chemistry. Another important area is molecular knot theory and circuit topology that describe the topology of folded linear molecules such as proteins and nucleic acids.
Stereochemistry in the context of Cyclic compound
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds with rings containing both carbon and non-carbon). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many billions.
- Cyclic compound examples: All-carbon (carbocyclic) and more complex natural cyclic compounds
-
 Cycloalkanes, the simplest carbocycles, including cyclopropane, cyclobutane, cyclopentane, and cyclohexane. Note, elsewhere an organic chemistry shorthand is used where hydrogen atoms are inferred as present to fill the carbon's valence of 4 (rather than their being shown explicitly).
Cycloalkanes, the simplest carbocycles, including cyclopropane, cyclobutane, cyclopentane, and cyclohexane. Note, elsewhere an organic chemistry shorthand is used where hydrogen atoms are inferred as present to fill the carbon's valence of 4 (rather than their being shown explicitly). -
 Ingenol, a complex, terpenoid natural product, related to but simpler than the paclitaxel that follows, which displays a complex ring structure including 3-, 5-, and 7-membered non-aromatic, carbocyclic rings.
Ingenol, a complex, terpenoid natural product, related to but simpler than the paclitaxel that follows, which displays a complex ring structure including 3-, 5-, and 7-membered non-aromatic, carbocyclic rings. -
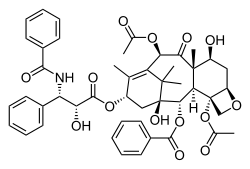 Paclitaxel, another complex, plant-derived terpenoid, also a natural product, displaying a complex multi-ring structure including 4-, 6-, and 8-membered rings (carbocyclic and heterocyclic, aromatic and non-aromatic).
Paclitaxel, another complex, plant-derived terpenoid, also a natural product, displaying a complex multi-ring structure including 4-, 6-, and 8-membered rings (carbocyclic and heterocyclic, aromatic and non-aromatic).
Adding to their complexity and number, closing of atoms into rings may lock particular atoms with distinct substitution (by functional groups) such that stereochemistry and chirality of the compound results, including some manifestations that are unique to rings (e.g., configurational isomers). As well, depending on ring size, the three-dimensional shapes of particular cyclic structures – typically rings of five atoms and larger – can vary and interconvert such that conformational isomerism is displayed. Indeed, the development of this important chemical concept arose historically in reference to cyclic compounds. Finally, cyclic compounds, because of the unique shapes, reactivities, properties, and bioactivities that they engender, are the majority of all molecules involved in the biochemistry, structure, and function of living organisms, and in man-made molecules such as drugs, pesticides, etc.


