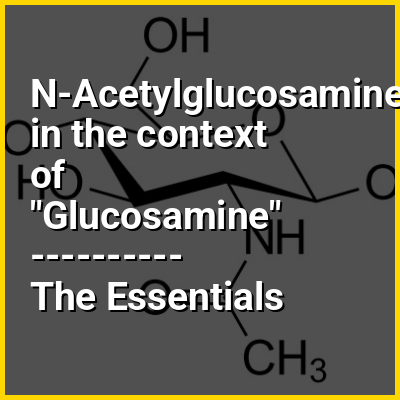
Microfungi or micromycetes are fungi—eukaryotic organisms such as molds, mildews and rusts—which have microscopic spore-producing structures. They exhibit tube tip-growth and have cell walls composed of chitin, a polymer of N-acetylglucosamine. Microfungi are a paraphyletic group, distinguished from macrofungi only by the absence of a large, multicellular fruiting body. They are ubiquitous in all terrestrial and freshwater and marine environments, and grow in plants, soil, water, insects, cattle rumens, hair, and skin. Most of the fungal body consists of microscopic threads, called hyphae, extending through the substrate in which it grows. The mycelia of microfungi produce spores that are carried by the air, spreading the fungus.
Many microfungi species are benign, existing as soil saprotrophs, for example, largely unobserved by humans. Many thousands of microfungal species occur in lichens, forming symbiotic relationships with algae. Other microfungi, such as those of the genera Penicillium, Aspergillus and Neurospora, were first discovered as molds causing spoilage of fruit and bread.
View the full Wikipedia page for Microfungi