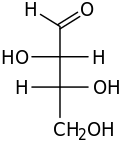
Monosaccharides (from Greek monos: single, sacchar: sugar), also called simple sugars, are a class of organic compounds usually with the formula (CH2O)x. By definition they have two or more carbon-carbon bonds. More specifically, they are classified as polyhydroxy aldehydes or polyhydroxy ketones with the respective formulas H-[CHOH]
n-CHO and H-[CHOH]
m-CO-[CHOH]
n-H. Monosaccharides can be classified by the number x of carbon atoms they contain: triose (3), tetrose (4), pentose (5), hexose (6), heptose (7), and so on.
They are colorless, water-soluble, and crystalline organic solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides).