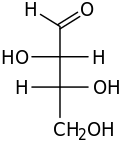
D-Erythrulose (also known as erythrulose) is a tetrose carbohydrate with the chemical formula C4H8O4. It has one ketone group and so is part of the ketose family. It is used in some self-tanning cosmetics, in general, combined with dihydroxyacetone (DHA).
Erythrulose/DHA reacts with the amino acids in the proteins of the first layers of skin (the stratum corneum and epidermis). One of the pathways involves free radicals at one of the steps of the Maillard reaction, distantly related to the browning effect when a cut apple slice is exposed to oxygen. The other pathway is the conventional Maillard reaction; both pathways are involved in the browning during food preparation and storage. This is not a stain or dye, but rather a chemical reaction that produces a color change on all treated skin. It does not involve the underlying skin pigmentation nor does it require exposure to ultraviolet light to initiate the color change. However, the 'tan' produced by erythrulose/DHA only has an SPF of up to 3, and enhances the free radical injury from UV (compared to untreated skin) for the 24 hours after self-tanner is applied, according to a 2007 study led by Katinka Jung of the Gematria Test Lab in Berlin. Forty minutes after the researchers treated skin samples with high levels of erythrulose, they found that more than 140 percent additional free radicals formed during sun exposure compared with untreated skin.