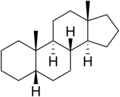
Gonane (cyclopentanoperhydrophenanthrene) is a chemical compound with formula C
17H
28, whose structure consists of four hydrocarbon rings fused together: three cyclohexane units and one cyclopentane. It can also be viewed as the result of fusing a cyclopentane molecule with a fully hydrogenated molecule of phenanthrene, hence the more descriptive name "perhydrocyclopenta[a]phenanthrene". The non-systematic version of the above name is "cyclopentanoperhydrophenanthrene".
It has no double bonds, that is, it is completely saturated and is considered the main structure of steroids, often referred to as the steroid nucleus. There are many forms of gonane, but only a few occur naturally in living organisms. Some common forms include 5α-gonane and 5β-gonane. Estrane, androstane, and pregnane are derivatives of gonane with additional methyl or ethyl groups attached to certain carbon positions. The term gonane is also used to describe a group of progestins that are similar to levonorgestrel but have a slightly different structure than other hormones like estranes.