In organic chemistry, an aldehyde (/ˈældɪhaɪd/) (lat. alcohol dehydrogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure R−CH=O. The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

- ⭐ Core Definition: Aldehyde
- Aldehyde in the context of Monosaccharide
- Aldehyde in the context of Glucose
- Aldehyde in the context of Organic redox reaction
- Aldehyde in the context of Formaldehyde
- Aldehyde in the context of Aldol reaction
- Aldehyde in the context of Arabinose
- Aldehyde in the context of Acetal
- Aldehyde in the context of Psittacofulvin
- Aldehyde in the context of Tetrose
- Aldehyde in the context of Heptose
- Aldehyde in the context of Sweetness
- Aldehyde in the context of Acyl
- Aldehyde in the context of Acetaldehyde
- Aldehyde in the context of Xylose
- Aldehyde in the context of Furfural
- Aldehyde in the context of Alcohol oxidation
- Aldehyde in the context of -ose
Aldehyde in the context of Monosaccharide
Monosaccharides (from Greek monos: single, sacchar: sugar), also called simple sugars, are a class of organic compounds usually with the formula (CH2O)x. By definition they have two or more carbon-carbon bonds. More specifically, they are classified as polyhydroxy aldehydes or polyhydroxy ketones with the respective formulas H-[CHOH]
n-CHO and H-[CHOH]
m-CO-[CHOH]
n-H. Monosaccharides can be classified by the number x of carbon atoms they contain: triose (3), tetrose (4), pentose (5), hexose (6), heptose (7), and so on.
They are colorless, water-soluble, and crystalline organic solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (though not all molecules with this formula are monosaccharides).
View the full Wikipedia page for MonosaccharideAldehyde in the context of Glucose
Glucose is a sugar with the molecular formula C6H12O6. It is the most abundant monosaccharide, a subcategory of carbohydrates. It is made from water and carbon dioxide during photosynthesis by plants and most algae. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. Glucose is often abbreviated as Glc.
In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is d-glucose, while its stereoisomer l-glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants. In animals, it is released from the breakdown of glycogen in a process known as glycogenolysis.
View the full Wikipedia page for GlucoseAldehyde in the context of Organic redox reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen. Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation:
When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidations electrons are removed and the electron density of a molecule is reduced. In reductions electron density increases when electrons are added to the molecule. This terminology is always centered on the organic compound. For example, it is usual to refer to the reduction of a ketone by lithium aluminium hydride, but not to the oxidation of lithium aluminium hydride by a ketone. Many oxidations involve removal of hydrogen atoms from the organic molecule, and reduction adds hydrogens to an organic molecule.
View the full Wikipedia page for Organic redox reactionAldehyde in the context of Formaldehyde
Formaldehyde (/fɔːrˈmældɪhaɪd/ for-MAL-di-hide, US also /fər-/ fər-) (systematic name methanal) is an organic compound with the chemical formula CH2O and structure H2C=O. The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as aqueous solutions (formalin), which consists mainly of the hydrate CH2(OH)2. It is the simplest of the aldehydes (R−CHO). As a precursor to many other materials and chemical compounds, in 2006 the global production of formaldehyde was estimated at 12 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings.
Formaldehyde also occurs naturally. It is derived from the degradation of serine, dimethylglycine, and lipids. Demethylases act by converting N-methyl groups to formaldehyde.
View the full Wikipedia page for FormaldehydeAldehyde in the context of Aldol reaction
The aldol reaction (aldol addition) is a reaction in organic chemistry that combines two carbonyl compounds (e.g. aldehydes or ketones) to form a new β-hydroxy carbonyl compound. Its simplest form might involve the nucleophilic addition of an enolized ketone to another:
These products are known as aldols, from the aldehyde + alcohol, a structural motif seen in many of the products. The use of aldehyde in the name comes from its history: aldehydes are more reactive than ketones, so that the reaction was discovered first with them.
View the full Wikipedia page for Aldol reactionAldehyde in the context of Arabinose
Arabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde (CHO) functional group.
View the full Wikipedia page for ArabinoseAldehyde in the context of Acetal
In organic chemistry, an acetal is a functional group with the connectivity R2C(OR')2. Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.
The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place of an R on the central carbon). The IUPAC originally deprecated the usage of the word ketal altogether, but has since reversed its decision. However, in contrast to historical usage, ketals are now a subset of acetals, a term that now encompasses both aldehyde- and ketone-derived structures.
View the full Wikipedia page for AcetalAldehyde in the context of Psittacofulvin
Psittacofulvin pigments, sometimes called psittacins, are responsible for the bright-red, orange, and yellow colors specific to parrots. In parrots, psittacofulvins are synthesized by a polyketide synthase enzyme that is expressed in growing feathers. They consist of linear polyenes terminated by an aldehyde group. There are five known psittacofulvin pigments - tetradecahexenal, hexadecaheptenal, octadecaoctenal and eicosanonenal, in addition to a fifth, currently-unidentified pigment found in the feathers of scarlet macaws. Colorful feathers with high levels of psittacofulvin resist feather-degrading Bacillus licheniformis better than white ones.
Both carotenoids and psittacofulvins have narrow-band absorbance spectra, reflecting pale yellow or red pigmentary colors, making them difficult to distinguish between using spectral measurements. However, there are differences between them when researched spectroscopically. The carotenoid and psittacofulvin yellows are very similar, but the red parrot pigment offers an advantage: it creates a more deep-red color when compared to astaxanthin, the pigment's counterpart in most other birds.
View the full Wikipedia page for PsittacofulvinAldehyde in the context of Tetrose
In organic chemistry, a tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde (−CH=O) functional group in position 1 (aldotetroses) or a ketone (>C=O) group in position 2 (ketotetroses).
-
 D-Erythrose
D-Erythrose -
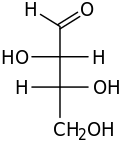 D-Threose
D-Threose -
 D-Erythrulose
D-Erythrulose
The aldotetroses have two chiral centers (asymmetric carbon atoms) and so 4 different stereoisomers are possible. There are two naturally occurring stereoisomers, the enantiomers of erythrose and threose having the D configuration but not the L enantiomers. The ketotetroses have one chiral center and, therefore, two possible stereoisomers: erythrulose (L- and D-form). Again, only the D enantiomer is naturally occurring.
View the full Wikipedia page for TetroseAldehyde in the context of Heptose
A heptose is a monosaccharide with seven carbon atoms.
They have either an aldehyde functional group in position 1 (aldoheptoses) or a ketone functional group in position 2, 3 or 4 (ketoheptoses). Ketoheptoses have 4 chiral centers, whereas aldoheptoses have 5.
View the full Wikipedia page for HeptoseAldehyde in the context of Sweetness
Sweetness is a basic taste most commonly perceived when eating foods rich in sugars. Sweet tastes are generally regarded as pleasurable. In addition to sugars like sucrose, many other chemical compounds are sweet, including aldehydes, ketones, and sugar alcohols. Some are sweet at very low concentrations, allowing their use as non-caloric sugar substitutes. Such non-sugar sweeteners include saccharin, aspartame, sucralose and stevia. Other compounds, such as miraculin, may alter perception of sweetness itself.
Sweetness is one of the five basic taste qualities and it mostly involves foods with sugars. It is known to be enjoyable and it is an important factor for food choices beyond cultures. Furthermore, sugars like sucrose, there are many other organic and inorganic compounds that bring out a sweet taste. This involves aldehydes, ketones, amino acids, and other artificial sweeteners. Sweetness recognition in our bodies takes part in an important role in energy control and evolutionary behavior.
View the full Wikipedia page for SweetnessAldehyde in the context of Acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group (R−C=O) or hydrogen in the case of formyl group (H−C=O). In organic chemistry, the acyl group (IUPAC name alkanoyl if the organyl group is alkyl) is usually derived from a carboxylic acid, in which case it has the formula R−C(=O)−, where R represents an organyl group or hydrogen. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond.
View the full Wikipedia page for AcylAldehyde in the context of Acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3CH=O. It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen. Acetaldehyde is "one of the most frequently found air toxins with cancer risk greater than one in a million".
View the full Wikipedia page for AcetaldehydeAldehyde in the context of Xylose
Xylose (cf. Ancient Greek: ξύλον, xylon, "wood") is a common monosaccharide, i.e. a simple sugar. Xylose is classified as aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group, at least in its open-chain form. It is abundant in biomass, and is one of the most abundant sugars in nature. It is a white, water-soluble solid.
View the full Wikipedia page for XyloseAldehyde in the context of Furfural
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs in a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word furfur, meaning bran, referring to its usual source. Furfural is derived only from dried biomass. In addition to ethanol, acetic acid, and sugar, furfural is one of the oldest known organic chemicals available readily purified from natural precursors.
View the full Wikipedia page for FurfuralAldehyde in the context of Alcohol oxidation
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.
View the full Wikipedia page for Alcohol oxidationAldehyde in the context of -ose
The suffix -ose (/oʊz, oʊs/) is used in organic chemistry to form the names of sugars. This Latin suffix means "full of", "abounding in", "given to", or "like". Numerous systems exist to name specific sugars more descriptively. The suffix is also used more generally in English to form adjectives from nouns, with the sense "full of", as in "verbose": wordy, full of words.
Monosaccharides, the simplest sugars, may be named according to the number of carbon atoms in each molecule of the sugar: pentose is a five-carbon monosaccharide, and hexose is a six-carbon monosaccharide. Aldehyde monosaccharides may be called aldoses; ketone monosaccharides may be called ketoses.
View the full Wikipedia page for -ose

