London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between atoms and molecules that are normally electrically symmetric; that is, the electrons are symmetrically distributed with respect to the nucleus. They are part of the van der Waals forces. The LDF is named after the German physicist Fritz London. They are the weakest of the intermolecular forces.
>>>PUT SHARE BUTTONS HERE<<<
London dispersion force in the context of Lipophilicity
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly") is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are called lipophilic (translated as "fat-loving" or "fat-liking"). Such non-polar solvents are themselves lipophilic, and the adage "like dissolves like" generally holds true. Thus lipophilic substances tend to dissolve in other lipophilic substances, whereas hydrophilic ("water-loving") substances tend to dissolve in water and other hydrophilic substances.
Lipophilicity, hydrophobicity, and non-polarity may describe the same tendency towards participation in the London dispersion force, as the terms are often used interchangeably. However, the terms "lipophilic" and "hydrophobic" are not synonymous, as can be seen with silicones and fluorocarbons, which are hydrophobic but not lipophilic.
London dispersion force in the context of Chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described as having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding.
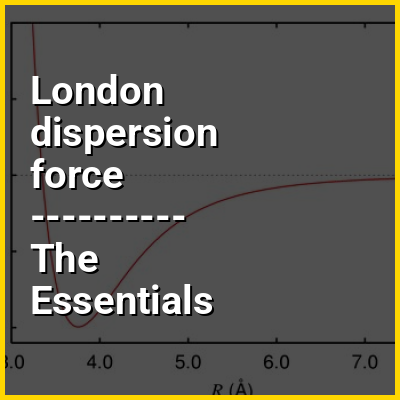
Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference" stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optimal distance (the bond distance) balancing attractive and repulsive effects explained quantitatively by quantum theory.
London dispersion force in the context of Noble gases
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some cases, oganesson (Og). Under standard conditions, the first six of these elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points. The properties of oganesson are uncertain.
The intermolecular force between noble gas atoms is the very weak London dispersion force, so their boiling points are all cryogenic, below 165 K (−108 °C; −163 °F).
London dispersion force in the context of Casimir effect
In quantum field theory, the Casimir effect (or Casimir force) is a physical force acting on the macroscopic boundaries of a confined space which arises from the quantum fluctuations of a field. The term Casimir pressure is sometimes used when it is described in units of force per unit area. It is named after the Dutch physicist Hendrik Casimir, who predicted the effect for electromagnetic systems in 1948.
In the same year Casimir, together with Dirk Polder, described a similar effect experienced by a neutral atom in the vicinity of a macroscopic interface which is called the Casimir–Polder force. Their result is a generalization of the London–van der Waals force and includes retardation due to the finite speed of light. The fundamental principles leading to the London–van der Waals force, the Casimir force, and the Casimir–Polder force can be formulated on the same footing.