The immunoglobulin heavy chain (IgH) is the large polypeptide subunit of an antibody (immunoglobulin). In the human genome, the IgH gene loci are on chromosome 14.
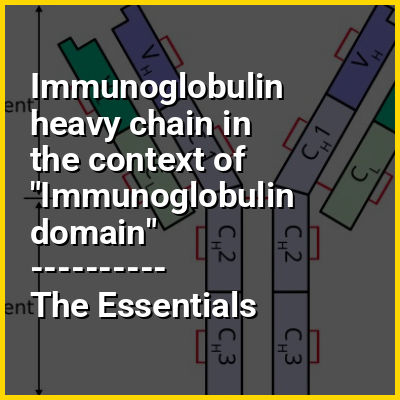
A typical antibody is composed of two immunoglobulin (Ig) heavy chains and two Ig light chains. Several different types of heavy chain exist that define the class or isotype of an antibody. These heavy chain types vary between different animals. All heavy chains contain a series of immunoglobulin domains, usually with one variable domain (VH) that is important for binding antigen and several constant domains (CH1, CH2, etc.). Production of a viable heavy chain is a key step in B cell maturation. If the heavy chain is able to bind to a surrogate light chain and move to the plasma membrane, then the developing B cell can begin producing its light chain.